|
Properties of
Equilibrium Constants
|
|
Equilibrium
Constants are...
|
|
Numbers
experimentally determined
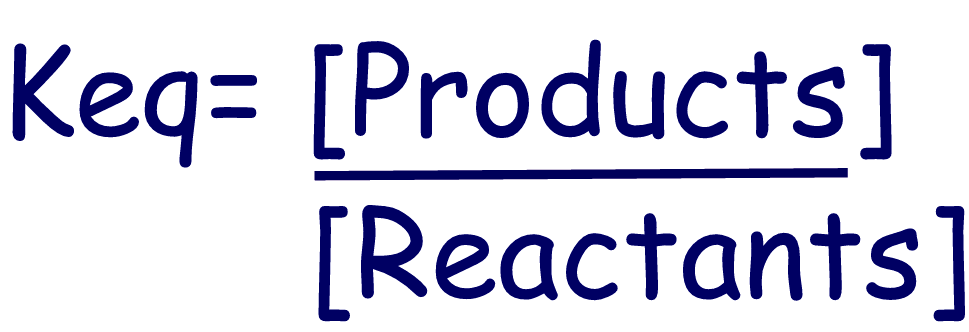
where [ ] represents
concentration in M |
Law of
Mass Action
(Law of Chemical Equilibrium)
For the following generic
equation…
aA
+ bB
⇄cC
+ dD
the
equilibrium constant would be…
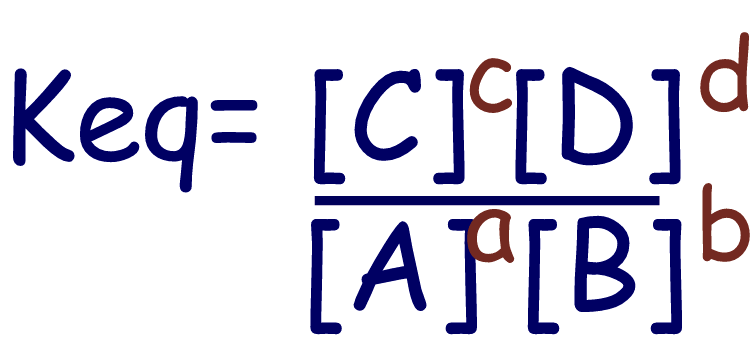 |
|
Dependent on
Temperature (two examples)
|
| Reaction |
Temperature (K) |
Keq |
|
H2+I2
⇄
2HI
http://www.vias.org/genchem/react_equilib_gasphase_12597_split004.html |
500 |
6.25×10-3 |
|
550 |
8.81×10-3 |
|
650 |
1.49×10-2 |
|
700 |
1.84×10-2 |
|
720 |
1.98×10-2 |
| Reaction |
Temperature (K) |
Keq |
|
N2
+ 3H2
⇄
2NH3
http://www.vias.org/genchem/react_equilib_gasphase_12597_split004.html
|
250 |
7×108 |
|
298 |
6×105 |
|
350 |
2×103 |
|
400 |
36 |
|
Include only (aq) or
(g) materials (solids
and liquids concentrations do not change)
|
| Reaction |
Keq |
|
N2(g)
+ 3H2(g)
⇄
2NH3(g) |
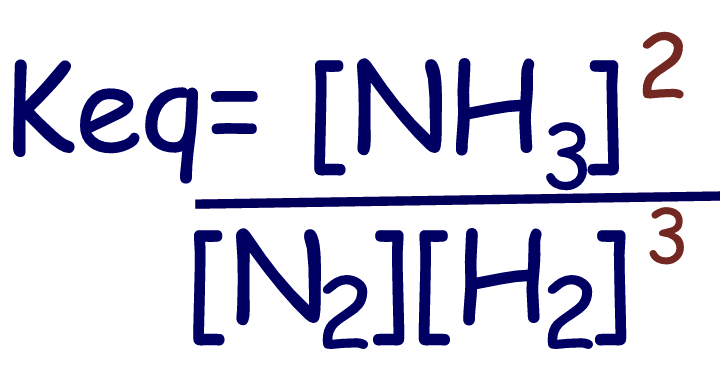 |
|
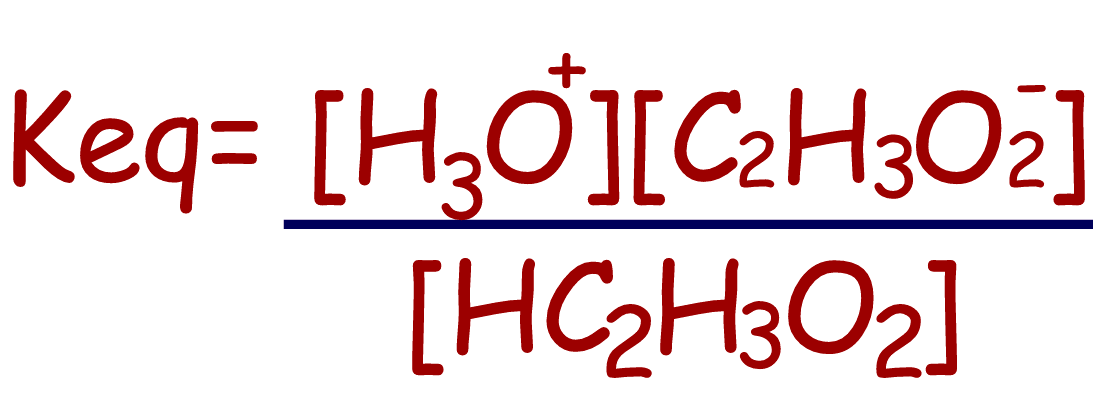
HC2H3O2(aq)
+ H2O(l)⇄
H3O+(aq)
+ C2H3O2-(aq) |
 |
|
Mg(s)
+ 2HCl(aq)
⇄
MgCl2(aq) + H2(g) |
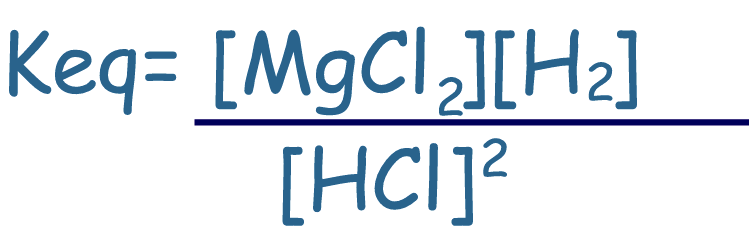 |
| C(s)
+ 2S(g)
⇄
1CS2(g) |
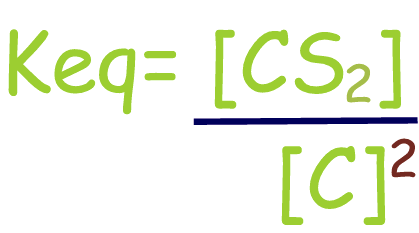 |
