|
Equilibrium:
What Is It?
|
|
|
Characteristics
of Equilibrium
|
- A
Dynamic State In Which Two Opposing Processes Take Place At The Same Time
And Rate
- Equal
rates... not necessarily equal amounts of reactants and products
- Systems
looks static as if nothing is happening
- System
at Equilibrium at the molecular level is dynamic, there is continual
change
|
|
|
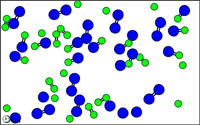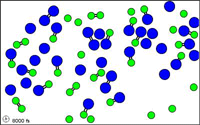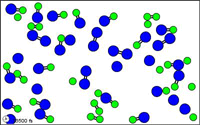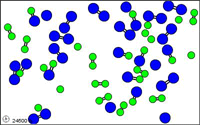
http://mw.concord.org/modeler1.3/mirror/chemistry/equilibrium.html
|
The animated gif shown here could be
representing the following equilibrium.
N2 +
O2  2 NO
2 NO
let N = blue
let O = green
Notice at all times
there are N2 molecules and O2 molecules and NO
molecules present.
|
|
Chemical
Equilibrium
N2+ 3H2 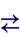 2NH3
2NH3
|
Examples
of
Equilibrium
|
Physical Equilbrium
Melting ice and
freezing ice at 0°C
H2O(l)
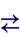 H2O(s) + energy
H2O(s) + energy
|
|
| 1.
When a restaurant get full, the number of tables being
seated = the number of tables being vacated. |
Some
Everyday Analogies of Equilibrium... |
| 2.
Athletic team substitutions such as line changes in
hockey... when one person goes off another goes in. |
| 3.
Carrying Capacity of an Ecosystem.... For every organism
that lives, a different one must die |
|
|
Symbols
Used To Describe Equilibrium |
System is at
Equilibrium |

|
|
Forward Reaction is
Favored |

|
|
Reverse Reaction is
Favored
|

|
