|
Bonds
and Energy
Energy changes are another aspect that can tied to
bonding changes.
| Bond
Change |
Energy
Change |
| Breaking Bonds
(both intermolecular and intramolecular bonds) |
Requires Energy |
| Forming Bonds |
Releases Energy |
Relating energy and bond changes to phase
changes....
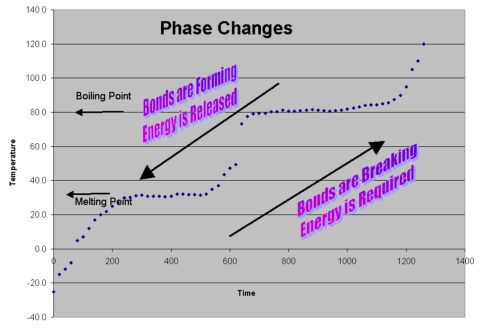
| Phase
change |
Transformation |
| Melting |
Solid +
energy Þ Liquid |
| Freezing |
Liquid Þ
Solid + energy |
| Evaporation |
Liquid + energy Þ
Gas |
| Condensation |
Gas Þ
Liquid + energy |
| Sublimation |
Solid + energy Þ
Gas |
Determining Energy Changes: In
Lab...
To describe these energy amounts released or
absorbed, we'll use the terms encountered previously, endothermic
(heat-absorbing) and exothermic (heat releasing). To measure the amount of
energy absorbed or released, we'll use the same equipment used for both chemical
(intramolecular bond) and physical (intermolecular bond) changes. The
device used for measuring energy changes is called a calorimeter. We'll
use two different units to measure energy.
| Energy
Unit |
Definition |
| calorie |
the amount of
energy it takes to heat up 1 gram of water 1° C |
| joule |
kg·m2/sec2 |
| It
should be noted that one calorie = 4.184 J |
Also
calories reported on food labels are big C Calories, meaning they are
kilocalories
A Snickers bar that is 300 Calories actually contains 300000 calories. |
Another concept that we will use to determine energy
changes in lab of specific heat capacity. The specific heat capacity of a
material (sometimes abbreviated cp is the amount of energy it takes to
heat 1 gram of the material, 1 °C.
Some common heat capacities that we will utilize are shown here...
| Substance |
Heat Capacity in
calories |
Heat Capacity in
joules |
| water (liquid) |
1 cal/gºC |
4.184 j/gºC |
| water (ice) at -5°C |
0.4964 cal/gºC |
2.077 j/gºC |
| water (steam) at
100°C |
0.4880 cal/gºC |
2.042 j/gºC |
| iron |
0.1073 cal/gºC |
0.449 j/gºC |
| copper |
0.09250 cal/gºC |
0.387 j/gºC |
| aluminum |
0.2144 cal/gºC |
0.897 j/gºC |
To literally calculate the energy released by a
change, we'll typically measure how much the temperature of the material
changes, the amount of the material involved, and it's characteristic specific
heat. To measure any multitude of changes, water is sometimes used to
indirectly interpret the energy change. Energy absorbed (or released) by
the material in question = energy released (or absorbed) by surrounding water.
| DH
= D temp
x mass x cp |
| Change in heat
energy (sometimes called the heat of reaction) = change in temperature x
mass x specific heat capacity |
| By definition,
exothermic reactions are assigned negative heats of reaction.
Endothermic reactions are assigned positive heats of reaction. |
Determining Energy Changes Using
Previous Lab Results: Hess' Law
The other way that we are going to determine
energy changes involved in physical and/or chemical transformations is to
utilize previously recorded heats of reaction data and a formula known as Hess'
law. People have recorded a number of different kinds of heats of
reactions. One can easily look up the energy released during combustion
reactions by looking up heats of
combustion data. The kind of heat reaction we'll be using is called
a heat of formation.
This is the amount of energy released or absorbed when elements react to form
the compound noted.
|
Hess' Law |
|
DH
= SHf products - SHf
reactants |
|
Heat of Reaction |
equals the sum of all of the
heats of formations for all of the products minus the sum of the heats of
formations for all of the reactants |
| It
should be noted that the heat of formations of elements in their natural
state is 0 calories or joules. |
| Heats
of reaction that are negative are exothermic. Positive heats of
reaction are endothermic. |
To see an example
of how heats of reaction are computed, using Hess' Law...
| 