Intermolecular versus Intramolecular
Bonds
Look at
the following diagram illustrating several molecules of water.
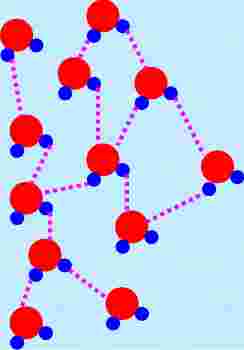 |
There are two different types of bonds shown in the diagram. One is called
an intramolecular (intra meaning within) bond. These connections are the
ones we studied previously as we worked to understand how atoms stick together,
specifically we called these these intramolecular bonds covalent bonds. (the
connections between the blue and red spheres.)
But as this picture is illustrating, the water molecule is not existing in
isolation, but rather is surrounded by other water molecules. There are
connections between these neighboring molecules. They are called
intermolecular (inter meaning between) bonds. Notice the arrangement of
the molecules and the atoms linked between molecules. (We will come back to that
idea later.) |
Intermolecular bonds - like these- determine
whether a material will be a solid, liquid or gas. This chart
summarizes the relationship between the degree and strength of intermolecular
bonding and the phase in which we find the material. The properties
that solids, liquids, and gases possess are also determined by these
intermolecular bonds.
Phase
Changes, Properties of Matter and Intermolecular Bonds
Gases are molecules that have gone solo.
Liquids and solids have varying degrees of intermolecular attractions
giving those phases their properties. Because of that, let's return to a
discussion on intermolecular bonding.
There are basically two types of intermolecular
bonds.
| Type of
Intermolecular Bond |
Characteristics |
| Dispersion
Interaction |
A
temporary weak attraction resulting from the uneven distribution of
electrons creating momentary + and - zones in the atom or
molecule. |
| Dipole-Dipole |
Interactions between
polar molecules, interactions between permanent + and - sites on molecules |
The fact that all atoms show dispersion
interaction intermolecular bonds implies that all atoms, even noble gas atoms
can at some point assume liquid or solid properties. Where that phase
transition takes place depends upon the strength of the intermolecular
bonds.
| Phenomenon |
Explanation |
| Melting
and Boiling Points |
Large
molecules will have higher melting and boiling points due to greater
dispersion interaction forces. Since large molecules have more
electrons, there is a greater dispersion interaction force.
Molecules with greater dipole interaction will have stronger
intermolecular bonds resulting in higher melting and boiling
points. |
| Capillary
Action |
Intermolecular
attractions (bonds) between different molecules |
| Density
of Water |
When
ice forms, the maximum number of intermolecular bonds between water
molecules form. |
| 